Lot’s of students who applied for Bihar Board Chemistry Compartmental exam they have given exam on the 07th July 2017 at the allotted examination center. Now want to check Answer of the BSEB 12th chemistry objective type questions. Some of the students who passed the exam want to see the Bihar Board Inter Compartmental Chemistry Objective type Question & Search on Google BSEB 12th Chemistry MCQ Question 2017.
If you are also searching for Bihar Board 10+2 Chemistry Objective & Subjective question then you will get here all BSEB Compartmental Examination CHEM Question MCQ and Subjective. Asked question is The Number of basic crystal system is, Freezing point, boiling point & colligative property of solution and all other question numbers wise given below.
Bihar Board Class 12th Compartmental Exam Chemistry Objective Question
Below www.resultfor.in is going to update all Bihar Board XIIth Class Chemistry Compartmental Exam Questions for the solution to the students. We also provide the answer key of all subject exam help of coaching centers.
Q 1. – The number of basic crystal system is –
(a) 4 (b) 6 (c) 7 (d) 8
Q2. In a crystal system AB which of the following crystal system will have parameters a≠ b ≠c and α = β= γ = 90·?
Q3. The freezing point of an aqueous solution is – 0.186⋅c. What is the evaluation of boiling point of this solution if Kr=1.86 and Kb = 0.512?
(a) 0.186 (b) -0.512 (c) 1.86 (d) 0.0512
Q4. Which of the following is not a colligative property of solution?
(a) Depression of freezing point (b) (c) (d)
(b) Elevation of Boiling point
(c) Relative lowering of vapor pressure (d)
(d) Optional activity
Q5. Faraday’s second law of electrolysis is related to –
(a) the atomic number of cation (b) Equivalent weight of electrolyte (c) atomic number of an anion (d) Speed of cation
Q6. The order of reaction of the reaction –
CH3COOC2H5 + H2O —H+ CH3COOH + C2H5OH is
(a) 3 (b) 2 (c) 1 (d) 0
Q7. A2O5 is good –
(a) Adsorbed (b) Absorbent (c) Reducing agent (d) Bleaching of color
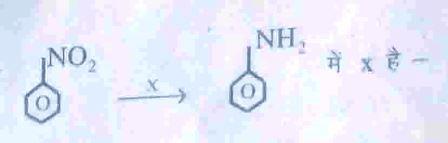
Q8. Which of the following method is used for the concentration of zinc blende ore ?
(a) Gravity separation process (b) Magnetic separation process (c) (d)
(c) Froth flotation process (d) None of these
Q9. The formula of copper pyrite is –
(a) CuFeS (b) CuFeS2 (c) Cu2S (d) Cu2FeS2
Q10. Which of the following is not a p – block element?
(a) Sn (b) P (c) S (d) Ba
Q11. Which among the following is the strongest acid?
(a) HF (b) HCI (c) HBr (d) HI
Q12. Which of the following is not a member of first transition elements series?
(a) Cr (b) Fe (c) Mg (d) Mn
Q13. The hybridization of Fe in K4 [fe (CN)6]
(a) dSp2 (b) Sp3 (c) d2Sp3 (d) Sp3d2
Q14. [Co (NH3)6] CI3 is which type of complex compound?
(a) Cationic complex (b) Anionic complex (c) Neutral complex (d) None of these
Here is all about the Bihar Board XIIth Class Chemistry Compartmental Exam Questions for the solution
Q15. In a given reaction –
CH3-CH2-CH = CH2 + HI → X the product is –
(a) 1- Iodobutane (b) 2- Isobutane (c) 3- Butene (d) 1,2- di- iodobutane
Q16. The reaction is given below –
(a) Kolbe’s reaction (b) Reimer Tiemann’s reaction
(c)Friedel craft’s reaction (d) Sandmeyer’s reaction
Q17. Glycerol is –
(a) Monohydric alcohol (b) Dihydric Alcohol (c) Trihydric alcohol (d) Primary alcohol
Q18. Cannizzaro’s reaction is given by-
(a) CH3 CHO (b) HCHO (c) CH3-CH2 – CHO (d) CH3-CH(CH3)- CHO
Q19. In a chemical reaction –
the product B is –
(a) Acetonitrile (b) Ethyl Cyanide (c) Methylamine (d) Acetamide
Q20. – The product formed by heating calcium formate is –
(a) Formaldehyde (b) Acetaldehyde (c) Acetone (d) Formic acid
Q21.
(a) Sn + NaOH (b) Sn/HCI (c) NH3 (d) All of these
Q22. Number of isomers formed by C3 H9 N is –
(a) 2 (b) 3 (c) 4 (d) 6
Q23. If n is number of moles of solute and N is number of moles of solvent the mole fraction strength of solute is –
Q24. Half life period of first order is independent of
(a) Initial concentration of reactant
(b) Temperature (c) Pressure (d) None of these
Q25. Which of the following is mono – saccharide?
(a) Sucrose (b) Maltose (c) Lactose (d) Fructose
Q26. Night blindness is caused by the deficiency of –
(a) Vitamin B 12 (b) Vitamin A (c) Vitamin C (d) Vitamin E
Q27. Natural rubber is a polymer of –
(a) Isoprene (b) Chloroprene (c) Butadiene (d) Styrene
Q 28. Dettol is used as –
(a) Antiseptic (b) Antipyretic (c) Analgesic (d) None of these
All above after the solve of this question answer is given the BSEB 12th Students. for exact calution of marks in Bihar Board Inter Compartmental Chemistry Marks solve by your teacher.
If you wish to anythings about the Answer key of Bihar Board Class 12th Chemistry Question Answer and solution. Send your feedback/Comments through the below box.
result kab ayeg
compartmental ka
Many of the answers of the question is wrong
Like question number 1 the answer is 7 not 8
Bihar board suplamenty ka result kab aayega sir
Hii. Sir.. I Wana say that.. Physics objective question answer upload in this site sir… Please I requested to you.. Tnk u sir